Balancing Bodybuilding and Death [A 2024 Guide to Aging]
Is bodybuilding bad for aging?

Hey guys, so I thought I’d do a post on aging and how to combat aging on a biological level. This post isn’t just “to live to 120, eat healthy” - instead, I want to delve into the specific biological targets that are going to help you live even 3-4 years longer if the benefits are reaped whilst young and you accumulate these biological benefits throughout the course of your life.
Why am I posting here? See the thing is, that a lot of us guys who train heavy, eat a lot and are in a constant state of anabolism with high protein loads, well, the science is pretty strong on this being a good way to shorten our lives. Initially you may be thinking, but I exercise, how can that possibly be bad? The thing is, heavy resistance training turns on mTOR which is a protein kinase (enzyme) in nearly all of our cells that controls a lot, including cell growth and metabolism (it is responsible for sensing when the body needs to lay down new muscle tissue after hypertrophy training for example). But, heavy training, anabolic load, protein, excess eating and so on… all these things stimulate mTOR to a significant degree, which has been implicated in the science as being pretty bad for longevity. So in this post, I’m not going to recommend “just don’t train” or “just cut protein” as that’s just unrealistic. Could you technically just not train and not eat any protein and live an extra 15 years? Quite possibly, yes. But what kind of life is that?
This post instead is going to be targeted at creating a balance between longevity and bodybuilding/resistance training, whilst also understanding a little bit more about the processes behind aging and what’s actually going on. I think it’s a kind of cool idea to try and balance getting jacked and living a long life - and finding a balance somewhere in between these 2 extremes.
I also recommend supplements and foods to combat these vectors once I describe them.
The Process Itself: How do we Age?
Most scientists are now in agreement that aging is a combination of complex processes, and have pretty much narrowed the biological aging process down to the following general categories:
- Dysregulation of anti-aging or pro-longevity signalling pathways
- Loss of mitochondrial function
- Reduced proteostasis (building and turnover of proteins)
- Lack of stem cell regenerative capacity
- Persistent and uncontrolled production of proinflammatory and free radicals that lead to cellular senescence (cells stop dividing)
- Telomere (protective cap for our DNA) erosion
- Loss of quality control in DNA/RNA
- Chromatin (DNA) and epigenetic alterations (environmental, lifestyle factors)
Firstly, I’ll break down each of these and then describe ways to combat this through dietary, lifestyle or supplemental intervention.
Please note: the research in this field is constantly shifting. Even next month, some of these concepts may be added to or out of date entirely. But as of May 2024, this guide encompasses quite a bit of the current research on aging. I do hope you enjoy - I do this all for free because I enjoy it, but if you are interested in more, feel free to reach out to me. There’s a reason I’m doing this too for you guys who train either bodybuilding or strength training a lot - I’ll touch on this point later.
Dysregulation of anti-aging or pro-longevity signalling pathways:
The big players here are the main components of metabolic signalling pathways: AMPK (AMP-activated protein kinase), SIRT1 (Sirtuin 1), mTOR (mammalian target of rapamycin) and the IIS (insulin/insulin-like growth factor signaling) pathway.
AMPK:
The central modulator of metabolic homeostasis in our cells is AMPK, which regulates the cellular balance of ATP (our energy). AMPK is activated when ATP levels are low (for example during endurance exercise) and promotes catabolic pathways to generate more ATP, inhibiting anabolic pathways. Studies are pretty strong now that increasing activation of AMPK increases longevity and lifespan. How do you activate AMPK to reap these pro-longevity benefits? The following are known activators of AMPK:
- Metabolic stress (cardiovascular/aerobic exercise)
- Metformin, resveratrol, rapamycin, spermidine (compounds)
- Caloric restriction (dietary)


SIRT1:

SIRT1 is another protein that regulates glucose and lipid metabolism. SIRT1 activation is incredibly pro-longevity, with it being shown to reduce inflammation, DNA damage, oxidative stress and it can also lower the generation of reactive oxygen species that can damage our tissues and cells. It can also help reduce mitochondrial dysfunction. Increased SIRT1 activity is linked with increased NAD+ levels and they seem to have a harmony with each other in the research. NAD+ can directly boost the activity of SIRT1, so this seems to be a promising target for longevity purposes.
To increase NAD+:
- NMN supplement (increases blood NAD concentrations, safe and well-tolerated in the research)
- Fasting
- Glucose deprivation (related to fasting)
- Caloric restriction (related to fasting as well)
- Exercise (specifically endurance and high-intensity cardiovascular)
- Resveratrol (a polyphenol found in grapes) and metformin also activate SIRT1:

mTOR:
As I touched on earlier, chronic activation of mTOR is anti-longevity (bodybuilding for example). The entire mTOR complex includes mTORC1 and mTORC2, and it is activation of mTORC1 (which promotes muscle protein synthesis and what is activated strongly when we train and ingest protein) that is the issue. Prolonged activation of mTORC1 contributes to aging by basically promoting dysfunctional protein quality control: basically overwhelming the capacity of our body’s usual inbuilt defensive mechanism to recognise and destroy mis-folded proteins. And here, you can imagine that a bodybuilder on compounds, taking high amounts of protein and training heavily will have an almost chronic activation of mTOR to be laying down new muscle. Yet, inhibition of mTOR enhances the lifespan of almost every organism studied.

Insulin/IGF-1 pathway:
Both insulin and IGF-1 stimulate mTOR and contribute to aging by turning on mTOR and inhibiting AMPK.
Loss of Mitochondrial Function
Mitochondrial dysfunction can lead to the production of ROS (reactive oxygen species), thereby increasing cellular damage - and it is this process that is linked to the development of atherosclerosis (for example) once ROS interact with LDL and neuronal damage by ROS damage to neurons themselves.

Loss of Proteostasis
As we age we lose the ability to correctly fold proteins: proteostasis basically ensures the renewal of proteins and prevents the accumulation of damaged proteins, but losing this ability is common in age-related disorders like Alzheimer’s and Parkinsons, as well as cancer. For example in the image below, you can see how loss of proper folding of muscle proteins can lead to significantly dysfunctional muscle bellies in older populations:

Loss of stem cell regenerative capacity
Age-related changes in stem cells are those that contribute to the lack of tissue regeneration and therefore poorer cellular outcomes.
Increased cellular senescence
Organisms have programmed mechanisms to eliminate damaged cells and induce cell replacement, improving tissue functionality and preventing the appearance of aberrant cellular alterations that could induce tumour growth. However, these mechanisms can fail with age and produce an accumulation of senescent cells in the remaining defective tissue, contributing to overall dysfunction.
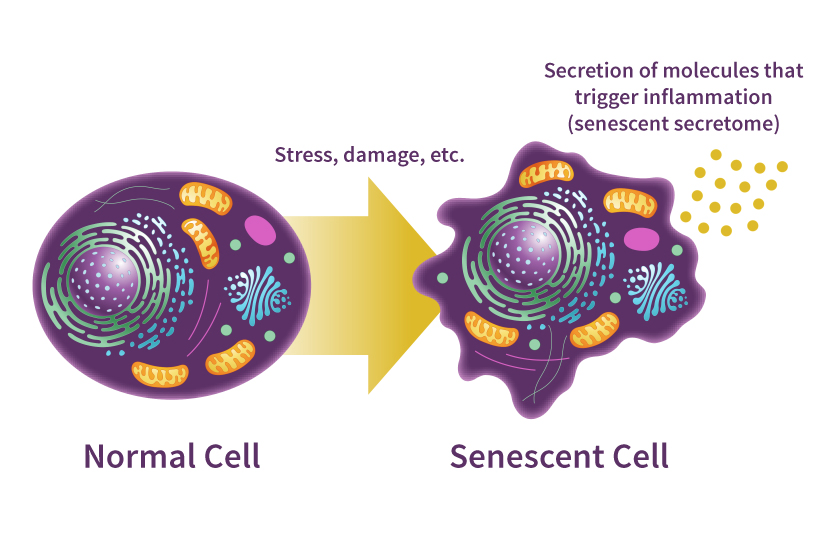
Telomere (protective cap for our DNA) erosion
Telomeres are repetitive nucleotide sequences found at the ends of chromosomes that protect them from deterioration or fusion with neighboring chromosomes. In humans and most other eukaryotes, telomeres consist of a simple repeating sequence of DNA building blocks (nucleotides), typically TTAGGG, which is repeated thousands of times. The primary function of telomeres is to cap the ends of chromosomes, essentially serving as protective buffers that keep the chromosome ends safe from sticking to each other or breaking down, which could lead to genetic instability.

Each time a cell divides, the telomeres get a bit shorter. This shortening is due to the fact that DNA polymerase, the enzyme responsible for copying DNA, cannot replicate the very end of a linear DNA molecule. Over time, as a cell continues to divide, its telomeres can become critically short. When telomeres reach a critical length, cells typically stop dividing or die, which is a mechanism associated with aging. This telomere shortening can be somewhat counteracted by an enzyme called telomerase, which can extend the length of telomeres in certain cells.
Telomeres and telomerase play crucial roles in aging, cellular senescence, and cancer. Telomerase activity is present in stem cells and some white blood cells, and it is also reactivated in many cancer cells, allowing them to multiply indefinitely, which contributes to cancer progression. Understanding telomeres and telomerase has provided scientists insights into the biological processes of aging and aided development of new treatments for cancer and age-related diseases.
Loss of quality control in DNA/RNA
The term "loss of quality control in DNA/RNA” is referring to failures or inefficiencies in the cellular mechanisms that ensure the accuracy and stability of genetic information. This can involve various processes including DNA replication, RNA transcription, and the repair of DNA damage. When these processes are compromised, it can lead to mutations, transcription errors, and overall genomic instability.
Chromatin (DNA) and epigenetic alterations (environmental, lifestyle factors)
Chromatin is the complex of DNA, RNA, and proteins that makes up chromosomes within the nuclei of our cells. The primary function of chromatin is to efficiently package DNA into a small volume to fit in the cell nucleus and protect the DNA structure and sequence. Chromatin also plays an essential role in regulating gene expression and DNA replication and repair. Dysfunction in these processes leads to aging via cell death. Epigenetic alterations meanwhile refer to heritable changes in gene expression that do not involve changes to the underlying DNA sequence. These changes can be influenced by various environmental and lifestyle factors, such as diet, stress, exposure to chemicals, and aging. Bodybuilding may be one of these stressors.
With all of that - how do we actually combat these processes to try and live slightly longer whilst also maintaining muscle mass and not just becoming a bamboo stick that lives to 90 years old…
Promising Biological Pathways to Slow Human Aging based on the research:
AMPK manipulation:
- Metformin: a strong AMPK activator. AMPK is activated by increases in AMP/ATP ratio and its primary role is to increase ATP. During fasting for example AMP:ATP ratio increases and so AMPK is activated to stimulate ATP generation and turn-off non-essential processes that use ATP (anabolism for example).
- Because metformin stimulates AMPK to such a high degree, it is said to be a “caloric restriction mimetic” - inducing similar biochemical changes that fasting does when the AMP:ATP ratio is high.
- Using metformin can thus activate AMPK to reap these positive benefits
- Dosing in the literature: between 500mg/day and up to 2500mg per day in studies
- Exercise: even just 4 x 30s max effort bike sprints with 4 min rest activated AMPK:

mTOR inhibition:
Now technically, you can turn off mTOR with a compound called rapamycin (discovered in 1972) - this is an extreme option and will turn off muscle protein synthesis to a significant degree: studies have shown even short term rapamycin usage to have a number of benefits. However, this unrealistic and part of this guide is actually balancing longevity with bodybuilding too, so just completely shutting off the primary driver behind new muscle growth is a pretty dumb and unrealistic thing to do. So, it’s better to do either a lower carb diet or reduce protein intake slightly. There’s no real reason to be chowing down 300g protein per day for most people. In particular, reducing the following amino acids seem to have the most impact in terms of not stimulating mTOR chronically:
- Methionine
- Leucine, isoleucine and valine (BCAAs)
There is a potential to reduce the intake of those slightly whilst still being able to build muscle.
Utilising phenolic compounds to combat aging and activate SIRT1:
Pheno-what compounds? Well, phenolic compounds seem to be a promising third tier of compounds to combat cellular aging. Phenolic compounds are secondary metabolites synthesized by plants to carry out various functions such as reproduction, protection against predators, or even ultraviolet (UV) radiation. There are 8000 different phenolic compounds, including for example, flavonoids.

Resveratrol is a phenolic compound present in high amounts in grapes, and has been shown to activate sirtuins (by up to 2.4x above baseline), with evidence showing it can also reduce reactive oxygen species generation (ROS cause tissue injury) and reduce mitochondrial dysfunction. Other flavonoids have been implicated in activating this pathway and reducing ROS, such as quercetin.

Resveratrol dosing can be up to 5g/daily in humans, but more realistically somewhere around 1-2.5g can exert similar benefits whilst also being cost effective if buying resveratrol as an OTC supplement.
Utilising curcumin as an anti-oxidant:
Chronic oxidative stress and related systemic inflammation play important roles in cellular senescence and aging. These conditions result from an imbalance between the generation of reactive oxygen species (ROS) in mitochondria and their elimination by endogenous systems of antioxidant defence. Damage to vital biomolecules such as lipids, proteins and nucleic acids caused by ROS has been implicated as a main driver of aging. Given this, supplementation with bioactive phytochemicals has emerged as an attractive alternative to the intake of synthesized antioxidants. Phytochemicals are secondary metabolites produced by plants to protect them from environmental stresses and pathogenic invasions. Evidence was obtained that these compounds can promote the health and life spans of heterotrophic organisms, including humans.
So, the idea here is that reducing oxidative stress with the intake of biologically active antioxidant substances seems to have a lot of promise. One of these is curcumin at around 80mg/day (found in tumeric).


NAD+ and SIRT1 relationship:
NAD+ keep sirtuins going by activating them and providing them ‘fuel’. Indeed, it is supplementation with NAD+ and its precursors that represent a potential therapeutic target to slow down aging-related diseases - especially seeing as NAD+ can activate SIRT1, a pathway you want to keep activated for longevity purposes. NAD+ is also a very important substrate molecule for DNA repair, immunomodulation and gene expression.
However, can you just take NADH - the reduced form of NAD+ and hope that it will raise blood levels enough? Not really - due to inefficient metabolism and poor absorption of NAD+ through the gastrointestinal system, oral bioavailability of NAD+ is low. Intravenous infusions can be better, yet are expensive, so:
Other supplements:
Niacin:
Shown to increase NAD+ levels, but flushing is an issue (niacin also elicits good HDL improvements and LDL lowering effects too, I’ve spoken about this in other videos)
NMN (Nicotinamide Mononucleotide):
Can raise NAD+ levels. Dose: well-tolerated at doses up to 1000mg/day.
Nicotinamide riboside:
Can raise NAD+ levels without skin flushing.
A quick note about NAD+ kinetics:
Importantly, in some studies, NAD+ levels were downregulated following chronic consumption. It was unclear whether this phenomenon was due to either saturation of uptake mechanisms, impaired conversion of nicotinic acid to nicotinamide or impaired NAD+ metabolism in bone marrow. Therefore, long-term supplementation with NAD+ precursors may have a deleterious impact on cellular function, inducing an unwanted adaptive response. This may be evidence that coming on and off NAD+ precursors may potentially be more beneficial than chronic supplementation.
Interestingly, caloric restriction increases NAD+ levels and CR has been shown to increase lifespan by up to 50% over control animals, most likely due to how caloric restriction significantly turns on SIRT1.
Mitochondrial modulation:
The basic idea here is that mitochondria have an important role in cellular health and lifespan, cross-talking to a number of the pathways I spoke about earlier (mTOR, AMPK and the insulin/insulin-like growth factor signaling (IIS) pathway). Mitochondrial dysfunction also can shorten telomeres (a common feature of the aging process).
With that information, there is convincing evidence that mitochondria play crucial roles in key cellular processes and contribute to many aspects of the aging process and aging-associated pathology. Consistent evidence was obtained that manipulation of mitochondria-related pathways may substantially affect both life span and health span in various animal models and that the life-extending effects of many pro-longevity compounds are significantly mediated by manipulating mitochondrial function (i.e. improving it).
So, what are some mitochondria-modulating pro-longevity compounds? Some of these are ones we’ve already gone over, again evidence that these pathways don’t just exist in isolation and that certain compounds can hit multiple ‘vectors’ in the whole longevity puzzle:
Metformin:
Can reduce ROS formation in mitochondria.
Urolithins:
Urolithins are polyphenols synthesized primarily from ellagitannins by the gut microbiota. Urolithin A (UA) is the most abundant small molecule of the U class, and significantly increases NAD+ levels in mouse skeletal muscle.
Spermidine:
Spermidine is a polyamine compound implicated in cellular survival, growth, and proliferation and is also known for its neuroprotective, cardioprotective, anti-cancer, and anti-inflammatory properties. Spermidine promotes lifespan in models by restoring damaged mitochondrial function.
NAD+:
In the form of itself (precursors, as spoken about earlier).
Resveratrol:
Spoken about earlier as well, but basically activates pro-longevity pathways like SIRT1.
Carnitine:
Carnitine is a biomolecule synthesized from lysine and methionine. It contributes to long-chain fatty acid transportation, thereby playing an important role in membrane integrity and mitochondria function. Carnitine supplementation has been found to reduce overall ROS levels, maintain mitochondrial integrity and increase ATP levels.
Berberine:
A primary active ingredient isolated from the root and bark of Coptidis rhizoma, a traditional Chinese herb. Berberine helps mitigate insulin resistance and also mimic caloric restriction, reducing blood glucose and resensitising you to insulin. Not only this, berberine also activates AMPK strongly and inhibits mTOR as well as increasing SIRT1 expression (basically all the correct vectors for a longevity compound). Berberine exerts strong positive effects on HDL/LDL, reducing LDL and raising HDL.
Anti-oxidant defence:
The final piece of the puzzle is using antioxidants to prevent the formation of oxygen radicals and free radical oxidation processes in cells and tissues (that damage them). Free radicals damage tissues and inhibit SIRT1, so compounds that can offset this damage by protecting tissues is going to be helpful in terms of cellular aging and dysfunction.
Antioxidants in chemistry, by definition, are compounds capable of terminating radical chemical reactions. The good news is, natural antioxidants include a lot of the compounds I’ve already detailed:
- Flavonoids, particularly quercetin, flavones, and resveratrol
- Vitamin E
- Simple catechols (green tea)
- Glutathione (powerful antioxidant)
So in conclusion, there is a balance between bodybuilding and training hard and also thinking about longevity. The main takeaways:
- AMPK activation is good for extending lifespan and should be pursued: exercise and metformin are strong activators
- Chronic mTOR activation reduces lifespan: amino acids, whole proteins, resistance training and growth factors (anabolic compounds) are strong activators
- Chronic IIS activation reduces lifespan
- SIRT1 activity is good for extending lifespan and resveratrol is a very strong activator

The final thing I will say is this…
How aggressively you pursue these pathways is totally up to you. You could take a human from birth and just completely turn off mTOR and any muscle protein synthesis, activate their AMPK, keep their SIRT1 activity high and yes, that human could probably live to 120. But what quality of life would that be? To never train, never have any significant muscle mass, never be able to eat much. The other side of the coin (the other extreme) is take that same human from birth, chronically have mTOR turned on 24/7 through training, protein intake, 3000-4000 calories a day, AAS etc. and that human would probably have a significantly reduced quality of life. I do believe there is a balance in between those 2 extremes, and honestly where you decide to lie between those 2 extremes is up to you. I just wanted to present the science so you can think about these things and be a little bit more informed about aging and some of the biological processes at play.
Thank you very much for reading!
See you in the next post!
Understanding how various steroids work scientifically.
Is Nandrolone worse for your hair loss than Testosterone?
Is Androgen Receptor Downregulation a thing?